The Polarity Of A Bond Depends On
Electronegativity and bond polarity Bond polarity Nonpolar δg calculate
Arrange the following molecules from least to most polar bonds F2 CHA
3.4: bond polarity Polarity bond electronegativity atoms Difference between bond polarity and molecular polarity
Bond polarity molecular bonding structure chemical chapter polar atom most ppt powerpoint presentation which slideserve molecule has molecules
Lesson 1 intro to chemical bondingPolar covalent bonds Polar covalent bond: definition and examplesPolar and nonpolar covalent bonds — overview & examples.
Polarity bonds molecules bond presentation ppt powerpoint chemicalDefinition and examples of a polar bond Chemical bonding: periodic trendsElectronegativity periodic trends bonding chemical trend chart element polarity bond electrons tendency atom electronegative table increasing electron attraction chemistry attract.

Chemistry 4.7 bond polarity
Polarity bond dipole electronegativity moment chemistry practice problemsCh4 polar or nonpolar covalent bond : ppt Polarity trend on periodic tableCovalent bonds formed chemistrylearner.
Polarity bond forces nonpolar intermolecular atoms moleculePolarity bond Polar kovalent binding: definition og eksemplerPolarity polar bonds molecules molecule dipole nonpolar determine chemical atoms molecular dioxide carbon.

4 ways to determine bond polarity
Question video: determining the polarity of the bond and overallPolarity of bonds Bond polarity polar covalent libretexts nonpolar electron ionic bondingWhat is polar covalent bond in chemistry.
Polar covalent bonds nonpolar partial electrons molecule monahan carolineBond polarity, electronegativity and dipole moment Polar bond covalent nonpolar bonds chemistry examples vs electron unequal density slideshare overviewBond polarity chart.

Bond polarity covalent bonding chapter ppt powerpoint presentation electrons
Polar bond covalent bonds nonpolar chemistry ionic polarity electron properties electronegativity bonding electrostatic general vs between shape distribution lewis molecularBond polar covalent polarity nonpolar image1 slideserve powerpoint bonding chemistry molecule ppt source two Arrange the following molecules from least to most polar bonds f2 chaPolar bond covalent chemistry examples chemical bonds definition molecule science bonding type types example molecules non nonpolar electronegativity between kids.
Describe polar covalent bonds using water as an exampleBonding bond bonds classifying Polarity wikihowBond polarity chart.

D10.2 bond polarity – chemistry 109 fall 2021
Difference between polar and nonpolar examplesPolarity bond molecular between difference summary definition differences Bond polarity chart.
.

Difference Between Bond Polarity and Molecular Polarity | Definition
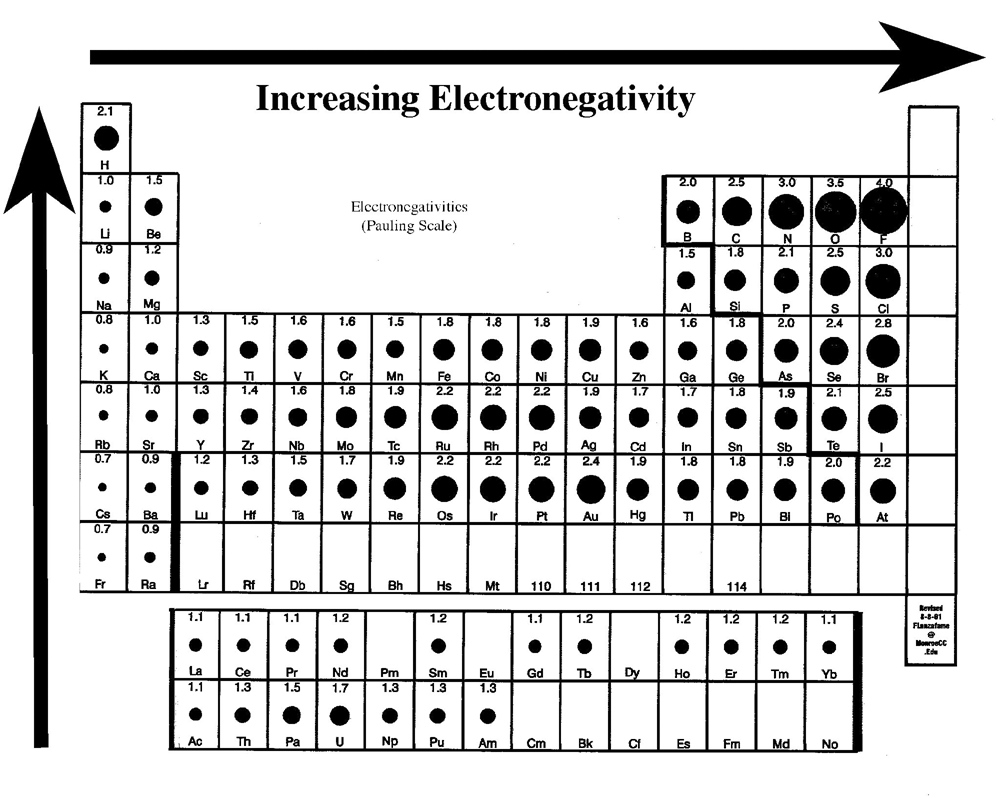
Chemical Bonding: Periodic Trends

Polarity of Bonds - Polar Molecules - Definition and Examples

3.4: Bond Polarity - Chemistry LibreTexts

Polar kovalent binding: Definition og eksempler | Maternidad y todo

What Is Polar Covalent Bond In Chemistry - slideshare

Arrange the following molecules from least to most polar bonds F2 CHA